This review contains the latest information related to inspection of manufacturers of veterinary medicinal products manufactured outside the Russian Federation for compliance with the requirements of the Good Manufacturing Practice (GMP). These inspections are conducted by specialists of the Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed” (FSBI “VGNKI”) subordinated to Rosselkhoznadzor.

GMP rules
Since January 2021, the GMP Rules of the Eurasian Economic Union (EAEU GMP Rules), approved by the Resolution of the EEC Council No. 77 of November 3, 2016, entered into force with respect to medicinal products for veterinary use [1].
In January 2021, on the Official Internet Portal of Legal Information publication.pravo.gov.ru the Order of Rosselkhoznadzor No. 1231 of November 17, 2020 “On approval of the application form for issuing a conclusion on the compliance for a manufacturer (foreign manufacturer) of veterinary medicinal products with the GMP Rules requirements, the form of the inspection report based on the results of the inspection of the manufacturer (foreign manufacturer) of veterinary medicinal products for compliance with the GMP Rules requirements and the form of the conclusion on the compliance of the manufacturer (foreign manufacturer) of veterinary medicinal products with the GMP Rules requirements” was published [2].
The approved document made changes to the form of the inspection report and the form of the conclusion on the compliance of the manufacturer of veterinary medicinal products with the GMP Rules requirements. In particular, in the form of the conclusion, the words “Rules of Good Manufacturing Practice, approved by order of the Ministry of Industry and Trade of the Russian Federation No. 916 of June 14, 2013” are replaced by the words “Rules of Good Manufacturing Practice of the Eurasian Economic Union, approved by the Resolution of the Council of the Eurasian Economic Commission Council No. 77 of November 3, 2016” [3].
In May 2021, the EEC Board approved a draft Resolution of the EEC Council on amendments to the EAEU GMP Rules [4].
According to the presented document, Annex 15 to the EAEU GMP Rules “Qualification and Validation” is presented in a new edition. The Resolution will enter into force 6 months after the date of its official publication [5].
The Annex establishes new, significantly expanded approaches to validation and qualification of manufacturing processes. For the first time, indications are introduced on the possibility of replacing validation with continuous process verification, ongoing process verification or verification (validation) of individual stages. Manufacturers will also be able to use a hybrid approach to validate the manufacturing process. As a result of the changes, the EAEU GMP Rules will be synchronized with the current European edition [6].
Additionally, you can read about the transition of veterinary manufacturers to the EAEU GMP Rules here: https://pharmprom.net/transition-of-veterinary-manufacturers-to-the-eaeu-gmp-rules/
Rules for organization and conduct of inspection
In April 2021, the EEC clarified that the provisions of the Commission’s acts concerning the order and procedure for conducting pharmaceutical inspections (Resolutions of the EEC Council of November 3, 2016 No. 82, 83, 90, 91) do not contain a corresponding clarification on the scope of application for veterinary medicinal products and therefore do not apply to their circulation. Considering that the draft Rules developed by the Commission, which provide for the order and procedure for conducting pharmaceutical inspections of the production of veterinary medicinal products, have not yet been adopted by the Commission, monitoring of compliance with the EAEU GMP Rules requirements in the production of veterinary medicinal products in the Member States of the Union is carried out in the manner established by the legislation of the corresponding Member State of the Union.
Thus, in relation to medicinal products for veterinary use, only the “Rules for organization and conduct of inspection of manufacturers of medicinal products for compliance with the GMP Rules requirements, as well as the issuance conclusions on the compliance of the manufacturer of medicinal products with the specified requirements”, approved by the Decree of the Government of the Russian Federation No. 1314 of December 03, 2015 “On determining the compliance of manufacturers of medicinal products with the GMP Rules requirements” apply [7].
Inspection results
In June 2021, within the framework of the business program of the exhibition “MVC: Cereals-Mixed Feed-Veterinary-2021”, the conference “Topical issues of circulation of veterinary medicinal products, prevention and treatment of infectious diseases of productive animals” was held. At this conference, Danil Rudniaev, Deputy Director, the Head of the Inspection Body of the FSBI “VGNKI” presented the topic “Four years of inspection of foreign manufacturers of veterinary medicinal products. Experience and Prospects”. Commenting on the results of inspections of foreign manufacturers in 2021, he noted that due to the ongoing pandemic, it is difficult to conduct on-site inspections and the queue of willing sites continues to grow. To date, it has been possible to carry out 14 inspections, of which only 4 were carried out with a visit to the manufacturing site [8].
In accordance with the inspection schedule published on the FSBI “VGNKI” website 05.07.2021, 50 inspections of manufacturers of veterinary medicinal products have already been planned for the second half of 2021 and for the first quarter of 2022, which sites located in Australia, Belarus, Belgium, Bulgaria, Brazil, Hungary, Vietnam, Germany, Egypt, Spain, Italy, China, Korea, the Netherlands, Slovenia, the USA, Turkey, Uruguay, Finland, France, Czech Republic and Switzerland [9].
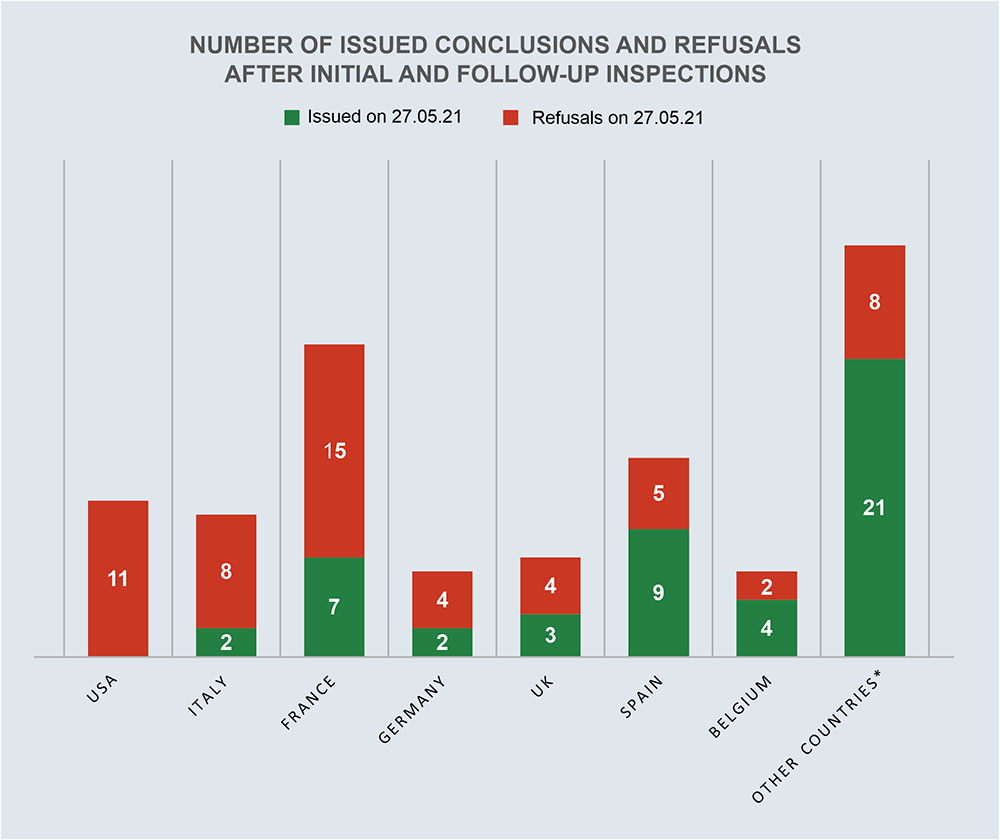
According to the register of issued conclusions published on the Rosselkhoznadzor website 27.05.2021, in 2021, 5 conclusions on the compliance of foreign manufacturers with the GMP Rules requirements have already been issued [10].
Two of these conclusions were made for the site located in France, and three others were made for sites located in Belgium, Israel and Portugal.
Information about French manufacturers of veterinary medicines and their inspection for compliance with the GMP Rules requirements is presented here: https://pharmprom.net/french-manufacturers-of-veterinary-medicines-and-their-inspection/
Remote inspection
In accordance with clause 7 (1). of the Decree of the Government of the Russian Federation No. 1314 of December 03, 2015, in conditions of the threat of the emergence, occurrence and liquidation of an emergency and (or) when there is a threat of the spread of a disease that poses a danger to others, diseases and injuries resulting from exposure to adverse chemical, biological, radiation factors, in which the inspection of the manufacturer, providing for an inspection of the manufacturing site is not possible, the conclusion issued for a period of 3 years, which is calculated from the date of completion of the inspection manufacturing site according to documents, including using remote interactions, including audio or video communication [7].
Speaking at the conference within the framework of the exhibition “MVC: Cereals-Mixed Feed-Veterinary-2021”, Danil Rudniaev said that in total 41 requests for remote inspections were received from foreign manufacturers of veterinary medicinal products to Rosselkhoznadzor, and in respect of 20 of them a positive decision was made (to date, 19 remote inspections have already been carried out and one inspection is scheduled for mid-July). Presenting the results of inspection of foreign manufacturers over the past year, he noted a drop in the number of inspections carried out, since due to the pandemic, the visit of inspectors to many sites did not take place. At the same time, Danil Rudniaev drew attention to the fact that it is impossible to inspect high-tech enterprises, especially those that produce immunobiological products, in a remote format [8].
Some recommendations for preparing of medicines manufacturers for remote inspection for compliance with the GMP Rules requirements can be found here (RU): https://pharmprom.ru/podgotovka-k-distancionnomu-gmp-inspektirovaniyu/
Corrective and preventive actions (CAPA) on the results of inspection
In accordance with clause 27. of the Decree of the Government of the Russian Federation No. 1314 of December 03, 2015, the foreign manufacturer or its authorized representative may submit to the authorized institution within 60 working days of receipt of the inspection report (without completing the section containing the final recommendations and conclusions), a letter accompanied by the CAPA plan as well as copies of documents, drawn up in the prescribed manner, containing measures for its implementation, along with a document confirming payment for the additional inspection services [7].
In June 2021, on the Rosselkhoznadzor website in the section “Inspection. Issuance of a conclusion” a new “Form for filling out a plan of corrective and preventive actions to eliminate nonconformities identified during the inspection” was published [11].
Frequently repeated nonconformities
In May 2021, a conference was held in Suzdal on the topic “Pharmaceutical quality system. Topical issues of quality assurance of medicinal products”. This event was held as part of the third day of the industry press tour of the III All-Russian Pharmprobeg. Speaking at this conference, Danil Rudniaev noted that the first thing the inspectors encountered when they began to inspect manufacturers of veterinary medicinal products was a large number of nonconformities associated with discrepancies in information in the registration dossier and the actual state of affairs on the site. For example, the control methods that were prescribed in the registration dossier did not match the control methods used at the enterprise [12].
At the conference within the framework of the exhibition “MVC: Cereals-Mixed Feed-Veterinary-2021”, Danil Rudniaev presented a list of frequently repeated nonconformities when inspecting manufacturers of veterinary medicines:
- change control is not carried out (the effectiveness of the changes made to the production process is not evaluated);
- there is no incoming control of primary packaging materials for microbiological purity (in particular, for non-sterile products);
- test samples are not representative of the batch of material or finished product from which they are selected (tools are used with which it is not possible to select a representative sample from large-volume raw material containers);
- qualification of warehouses is carried out without taking into account the risk of temperature fluctuations during the change of seasons (tests are not carried out during the hottest and coldest period of the year for a particular region);
- the quality control methods used at the enterprise are not validated (methods are not validated for specific premises and specific site equipment);
- stages of qualification of the performance or operation of premises, systems and equipment does not include the necessary elements (IQ, OQ, PQ);
- process validation does not perform tests at operating parameters equal to the upper and lower allowable limits;
- process validation (including cleaning validation) did not include 3 successive consecutive batches (cycles), at which the parameters were within the established limits;
- the intervals between use and cleaning as well as cleaning and reuse are not determined (the same for sterilization);
- the integrity control of vials with sterile products is not carried out (control is not carried out with the frequency determined taking into account the risk analysis);
- the primary containers of parenteral products are not checked for foreign matter (100% control of all units is not carried out) [8].
Agreeing of the timeframes for elimination of critical nonconformities
In May 2021, on the Official Internet Portal of Legal Information publication.pravo.gov.ru the Order of Rosselkhoznadzor No. 282 of March 22, 2021 “On the establishment of the procedure for the suspension of the sale and use of medicinal products for veterinary use” was published [13].
According to the approved document, one of grounds for consideration by Rosselkhoznadzor of the issue of suspending the sale and use of a medicinal product for veterinary use is the following case: the manufacturer of medicinal products, in a time agreed with Rosselkhoznadzor, has not eliminated violations of the GMP Rules requirements that were identified during the inspection of the manufacturer of medicinal products and that have led or may lead to production medicinal products that have caused or are likely to harm to life or health of animals [14].
In June 2021, in order to discuss issues related to the entry into force of the Order of Rosselkhoznadzor No. 282 of March 22, 2021, the Directorate for Domestic Veterinary Surveillance of Rosselkhoznadzor held a video conference with the participation of representatives of the veterinary and pharmaceutical business communities. Based on the results of the agreements reached at this meeting, on the Rosselkhoznadzor website, in the section “Pharmacovigilance”, “Recommendations for agreeing with Rosselkhoznadzor a plan of corrective and preventive actions to eliminate nonconformities identified during the inspection. Form for filling out a plan of corrective and preventive actions to eliminate nonconformities identified during the inspection” were published [15].
GMP-conclusion when putting medicines into civil circulation
In June 2021, at a plenary session of the State Duma of the Federal Assembly of the Russian Federation, a draft Federal Law “On amendments to the Federal Law “On circulation of medicines” on the issue of putting veterinary medicinal products into civil circulation” was adopted in the third reading. The adopted Federal Law No. 317-FZ of July 02, 2021 was published on the Official Internet Portal of Legal Information publication.pravo.gov.ru [16].
The Law stipulates that the entering into civil circulation of a medicinal product for veterinary use imported (transferred) to the Russian Federation is carried out if there is a conclusion on the compliance of the manufacturer of medicinal products with the GMP Rules requirements, which was issued by the authorized federal executive body for the manufacturing site of the medicinal product for veterinary use entering into civil circulation. The Federal Law will enter into force on September 1, 2023 [17].
Traning
In April 2021, the grand opening of the Eurasian Academy of Good Practices took place at the Chamber of Commerce and Industry of the Russian Federation, where both pharmaceutical inspectorates from different countries and specialized industry representatives will be trained. The opening ceremony was attended by the top officials of the relevant ministries and departments of the EAEU Member States, the EEC, representatives of the regulatory bodies of the union states, industry communities and associations, as well as heads of pharmaceutical companies. The Academy is a subsidiary the Federal State Institution “State Institute of Drugs and Good Practices” (FSI “SID & GP”) [18].
Speaking at the opening ceremony, Leonid Kish, Director of the FSBI “VGNKI”, noted that the creation of the Academy of Good Practices is a serious event not only in the field of advanced training of pharmaceutical inspectors but also in the preparation of new highly qualified specialists in the pharmaceutical industry [19].
At the conference within the framework of the III All-Russian Pharmprobeg, Vasilina Gritsyuk, Deputy Director of the FSBI “VGNKI” drew particular attention to the fact that even if there is a certain set of knowledge, it is extremely important to constantly improve qualifications [12].
In 2021, FSBI “VGNKI” continues to conduct training events for specialists of enterprises-manufacturers of veterinary medicines [20].
In the first half of 2021, as part of a joint program of promoting implementation of the best practices in the Russian pharmaceutical industry – the “SOLIDARY TRAINING PROGRAM” project, more than 20 corporate seminars were held for companies with an “open-door policy”. The project was initiated by the “PHARMSTRATEGY” company and FSI “SID & GP” [21].
In June 2021, registration was opened for the VI All Russia GMP Conference with international participation, which will be held in St. Petersburg on September 22-24. The organizer of the conference is the Ministry of Industry and Trade of Russia together with the FSI “SID & GP”. On September 23, within the framework of the conference, a panel discussion “Inspection of manufacturers of veterinary medicines. Regulation and statistics. The view of the state and business” will be held. Among the issues planned for the discussion: regulation of inspection of manufacturers of veterinary medicines under EAEU Rules; experience of inspecting European manufacturers of veterinary medicines. Among the speakers who are planning to speak are representatives of the FSBI “VGNKI”, the Ministry of Agriculture of Russia, Rosselkhoznadzor, the EEC, veterinary medicines manufacturing companies [22].
Manufacturers of veterinary medicines are encouraged to take an active part in training events and prepare more thoroughly for GMP inspections.
The material presented was prepared using data relevant to 21.07.2021.
In case of new or additional data, the article can be updated.
References:
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 03.11.2016 г. № 77 «Об утверждении Правил надлежащей производственной практики Евразийского экономического союза».
- Официальный интернет-портал правовой информации / Приказ Федеральной службы по ветеринарному и фитосанитарному надзору от 17.11.2020 № 1231 «О внесении изменений в приказ Россельхознадзора от 18 октября 2016 г. № 755 «Об утверждении формы заявления о выдаче заключения о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики, формы инспекционного отчета по результатам инспектирования производителя (иностранного производителя) лекарственных средств для ветеринарного применения на соответствие требованиям правил надлежащей производственной практики и формы заключения о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики». URL: http://publication.pravo.gov.ru/Document/View/0001202101180011 (дата обращения 15.07.2021)
- ФармПром.РФ / Новости / Изменились формы инспекционных отчетов и заключений по GMP для ветпроизводителей. URL: https://pharmprom.ru/izmenilis-formy-inspekcionnyx-otchetov-i-zaklyuchenij-po-gmp-dlya-vetproizvoditelej/ (дата обращения 15.07.2021)
- Евразийский экономический союз / Распоряжение Коллегии Евразийской экономической комиссии от 19.05.2021 г. № 77 «О проекте Решения Совета Евразийской экономической комиссии «О внесении изменений в Правила надлежащей производственной практики Евразийского экономического союза». URL: https://docs.eaeunion.org/docs/ru-ru/01429302/err_24052021_77 (дата обращения 15.07.2021)
- ФармПром.РФ / Новости / В Правилах GMP ЕАЭС изменятся требования к квалификации и валидации. URL: https://pharmprom.ru/v-pravilax-gmp-eaes-izmenyatsya-trebovaniya-k-kvalifikacii-i-validacii/ (дата обращения 15.07.2021)
- Евразийский экономический союз / Новости / Расширены возможности производителей лекарств по проведению валидации и квалификации производств в системе GMP. URL: https://eec.eaeunion.org/news/rasshireny-vozmozhnosti-proizvoditelej-lekarstv-po-provedeniyu-validatsii-i-kvalifikatsii-proizvodstv-v-sisteme-gmp/ (дата обращения 15.07.2021)
- ФГБУ «ВГНКИ» / Структура / Отдел инспекции производства на соответствие требованиям надлежащей производственной практики / Нормативно-правовая документация. URL: http://www.vgnki.ru/assets/files/post-1314.pdf (дата обращения 15.07.2021)
- ФармПром.РФ / Статьи / Четыре года инспектирования иностранных ветпроизводителей: опыт и перспективы. URL: https://pharmprom.ru/chetyre-goda-inspektirovaniya-inostrannyx-vetproizvoditelej-opyt-i-perspektivy-2/ (дата обращения 15.07.2021)
- ФГБУ «ВГНКИ» / Структура / Отдел инспекции производства на соответствие требованиям надлежащей производственной практики / График проведения инспектирования иностранных производителей на соответствие требованиям надлежащей производственной практики. URL: http://www.vgnki.ru/assets/files/grafik-na-sajt- pdf (дата обращения 05.07.2021)
- Россельхознадзор / Регистрация и лицензирование / Инспектирование. Выдача заключения / Реестр заключений о соответствии производителя требованиям правил надлежащей производственной практики. URL: https://fsvps.ru/fsvps/regLicensing/conclusion/conclusionReestr.html (дата обращения 15.07.2021)
- Россельхознадзор / Регистрация и лицензирование / Инспектирование. Выдача заключения / Форма заполнения плана корректирующих и предупреждающих действий по устранению несоответствий, выявленных в ходе инспектирования. URL: https://fsvps.gov.ru/fsvps/regLicensing/conclusion (дата обращения 07.2021)
- Фармпробег / Новости / Фармпробег-2021 в Суздале поднял тему фармацевтической системы качества. URL: https://pharmprobeg.ru/novosti/farmprobeg-2021-v-suzdale-podnyal-temu-farmatsevticheskoj-sistemy-kachestva/ (дата обращения 15.07.2021)
- Официальный интернет-портал правовой информации / Приказ Федеральной службы по ветеринарному и фитосанитарному надзору от 22.03.2021 № 282 «Об установлении Порядка приостановления реализации и применения лекарственных препаратов для ветеринарного применения». URL: http://publication.pravo.gov.ru/Document/View/0001202105200021 (дата обращения 15.07.2021)
- ФармПром.РФ / Новости / Утверждён порядок приостановки реализации и применения ветеринарных препаратов. URL: https://pharmprom.ru/utverzhdyon-poryadok-priostanovki-realizacii-i-primeneniya-veterinarnyx-preparatov/ (дата обращения 15.07.2021)
- Россельхознадзор / Регистрация и лицензирование / Фармаконадзор / Рекомендации по согласованию с Россельхознадзором сроков по устранению несоответствий, выявленных при проведении инспектирования/лицензионного контроля. Форма заполнения плана корректирующих и предупреждающих действий по устранению несоответствий, выявленных в ходе инспектирования/лицензионного контроля. URL: https://fsvps.gov.ru/fsvps/regLicensing/farmakonadzor.html (дата обращения 15.07.2021)
- Официальный интернет-портал правовой информации / Федеральный закон от 02.07.2021 № 317-ФЗ «О внесении изменений в Федеральный закон «Об обращении лекарственных средств». URL: http://publication.pravo.gov.ru/Document/View/0001202107020024 (дата обращения 15.07.2021)
- ФармПром.РФ / Новости / Закон о вводе в оборот ветеринарных препаратов принят Госдумой. URL: https://pharmprom.ru/zakon-o-vvode-v-oborot-veterinarnyx-preparatov-prinyat-gosdumoj/ (дата обращения 15.07.2021)
- Евразийская Академия надлежащих практик / Новости / Открытие GxP Академии объединило регуляторов стран ЕАЭС. URL: https://gxp-academy.org/news/otkrytie-gxp-akademii-obedinilo-regulyatorov-stran-eaes/ (дата обращения 15.07.2021)
- ФГБУ «ВГНКИ» / Пресс-центр / Новости / Директор ФГБУ «ВГНКИ» Леонид Киш принял участие в открытии Евразийской Академии надлежащих практик. URL: http://www.vgnki.ru/direktor-fgbu-vgnki-leonid-kish-prinyal-uchastie-v-otkrytii-evrazijskoj-akademii-nadlezhashhih-praktik.html (дата обращения 15.07.2021)
- ФГБУ «ВГНКИ» / Образование / Дополнительное профессиональное образование / Планы мероприятий / План обучающих мероприятий для специалистов предприятий-производителей лекарственных средств для ветеринарного применения на 2021 год. URL: http://www.vgnki.ru/assets/files/plan-2021-itog_proizvoditeli.pdf (дата обращения 15.07.2021)
- Совместная программа содействия внедрению лучших образцов надлежащих практик в российской фармацевтической отрасли / Отчеты / Календарно-тематический план «Солидарной программы обучения» (январь-июнь 2021 г.). URL: https://www.goodpractices.ru/upload/iblock/ca6/SP-i-SORpdf (дата обращения 15.07.2021)
- VI Всероссийская GMP-конференция с международным участием. URL: http://gosgmp.ru/ (дата обращения 21.07.2021)